Specialised Biotech/Pharma AI Solution for IND/CTA writings
Built by a group of seasoned industry experts with over 100+ years of experience in Regulatory Affairs, Pharmacology, DMPK, Toxicology, CMC, Medical Writing, and Clinical success.
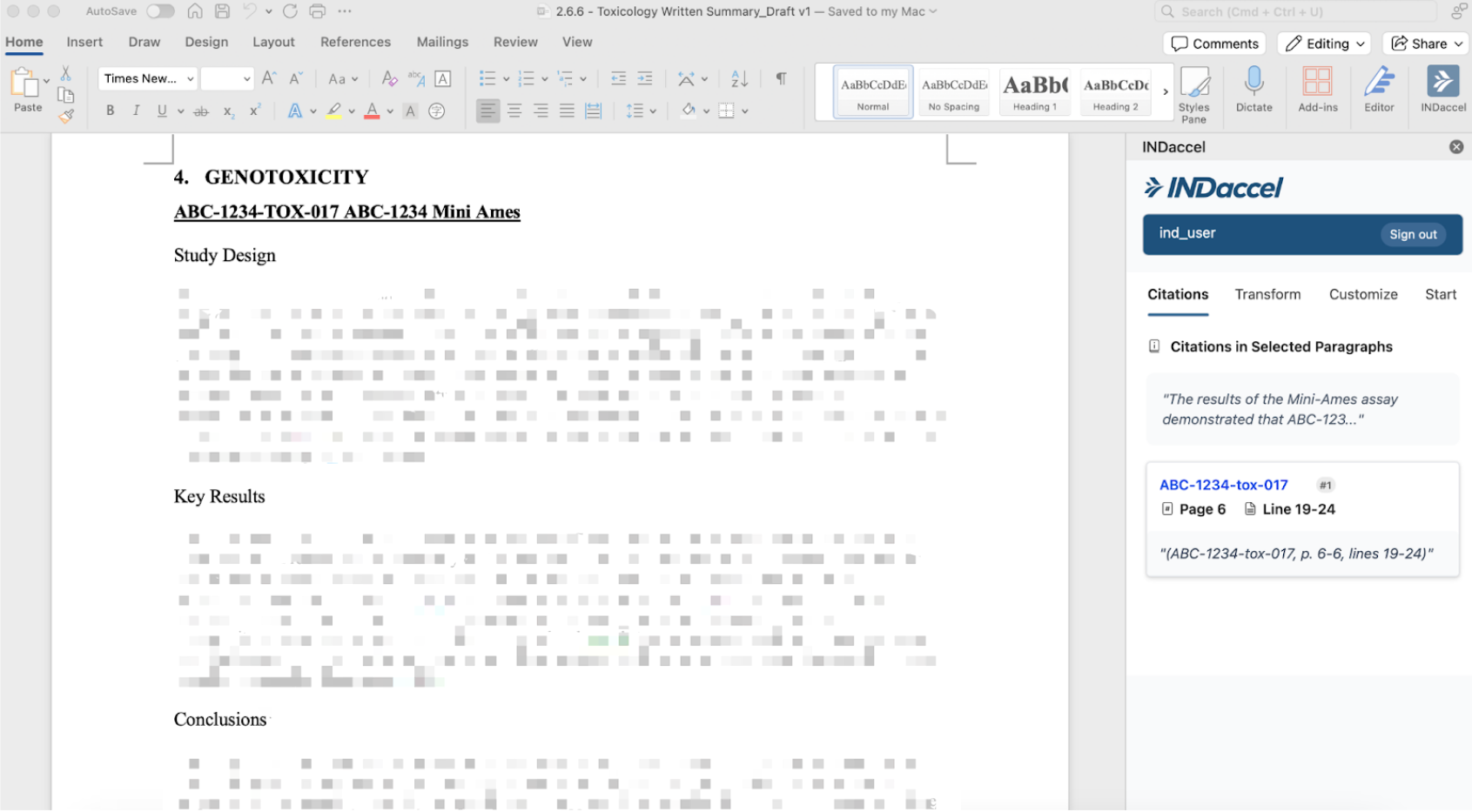
With a core focus on accuracy, efficiency, and traceability, INDaccel automates the most time-consuming aspects of regulatory document creation, streamlines IND workflows, accelerates IND submission timelines, reduces QC effort, and ensures high-quality, compliant documentation.
Features
Ensure Accuracy and Compliance with Source Traceability
Our platform enhances regulatory document quality by linking directly to source materials, enabling fast and reliable quality control.
Strengthen compliance and traceability
Efficient QC and review processes
AI-Powered Language Refinement from Source
Our platform enables draft documents connected directly to the source articles to revise content with precision. With AI-assisted suggestions, grounded in original data, our platform helps your scientific team members an efficient review or rewrite while maintaining clarity, consistency, and regulatory compliance throughout the process.
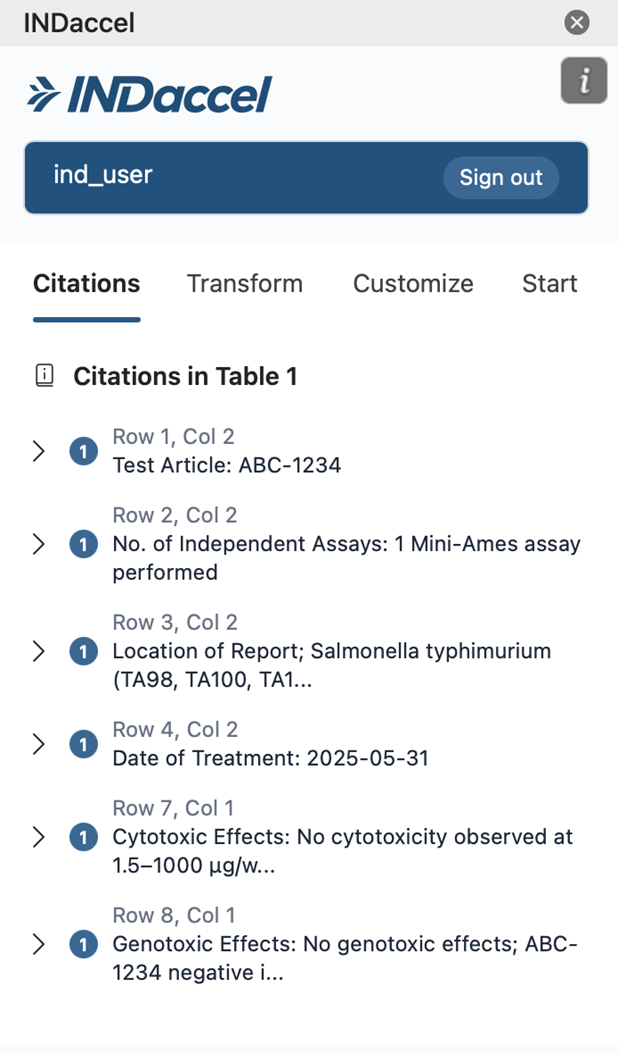
Citations
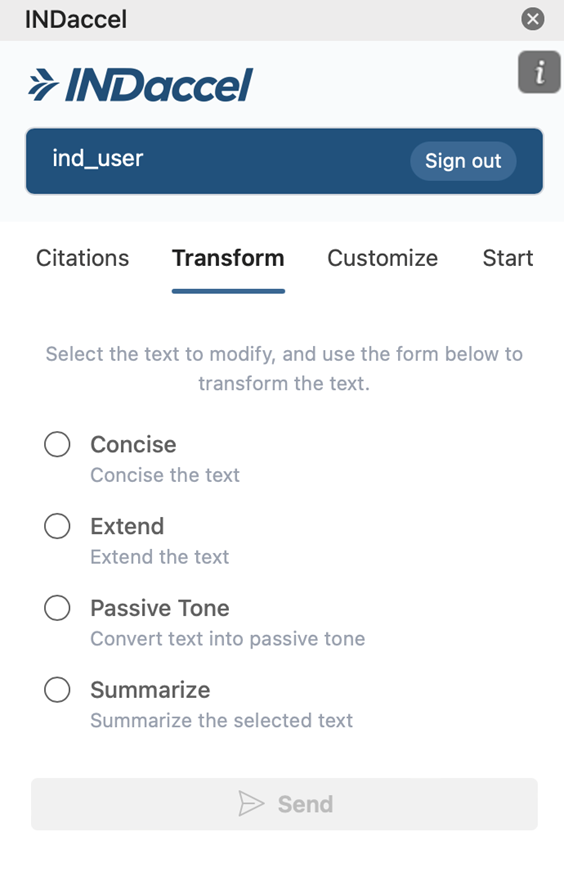
Transform
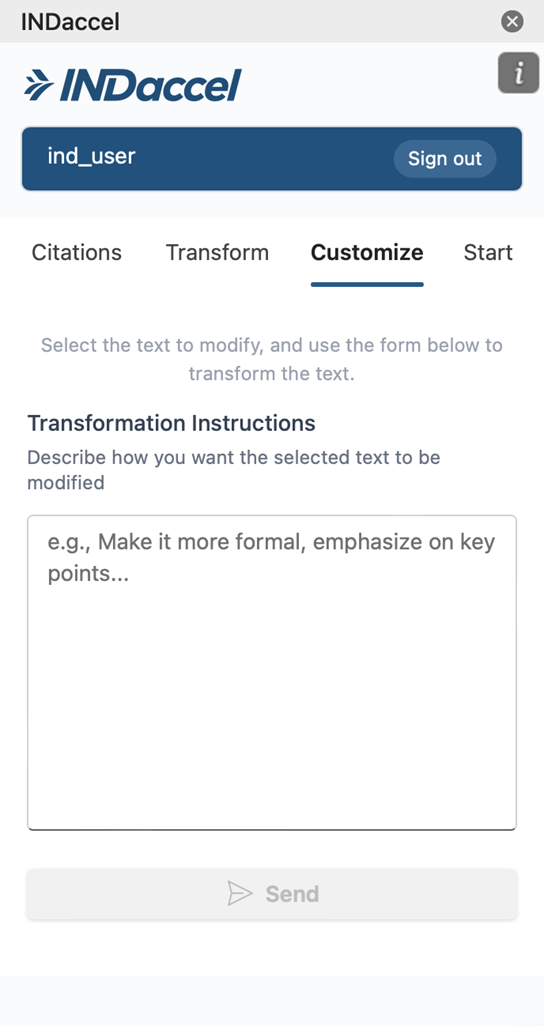
Customize
Privacy & Security
At INDaccel, we are committed to protecting your data with the highest standards of security and transparency.
No AI Training on User Data
We never use customer data to train AI models. Your information remains private and is never repurposed for machine learning.
Zero Data Retention
We do not retain user data longer than necessary. Data is only stored as required to deliver our Services or comply with legal obligations. Upon completion of your project, your data will be deleted, and an audit trail report will be provided for your records.
Data Isolation
All data remains confidential and is securely stored in a dedicated, separate server that is compliant with your company's security standards. Our platform ensures data security by strictly isolating your data into your own dedicated server.
Security Best Practices
We implement biotech industry-standard security measures to keep your data safe, private, and protected within your account.
Minimal Data Sharing
We share data only with absolutely necessary, industry-standard trusted service providers (e.g., Google Cloud Platform for hosting) to deliver services. We do not sell your personal information.
Audit Trial Report
Upon request, we can provide a comprehensive audit trail report at the completion of your project. This report documents how your data was accessed, processed, and deleted, ensuring full transparency and accountability. It serves as verifiable proof that your data lifecycle has been handled in accordance with our zero-data-retention policy and security standards.